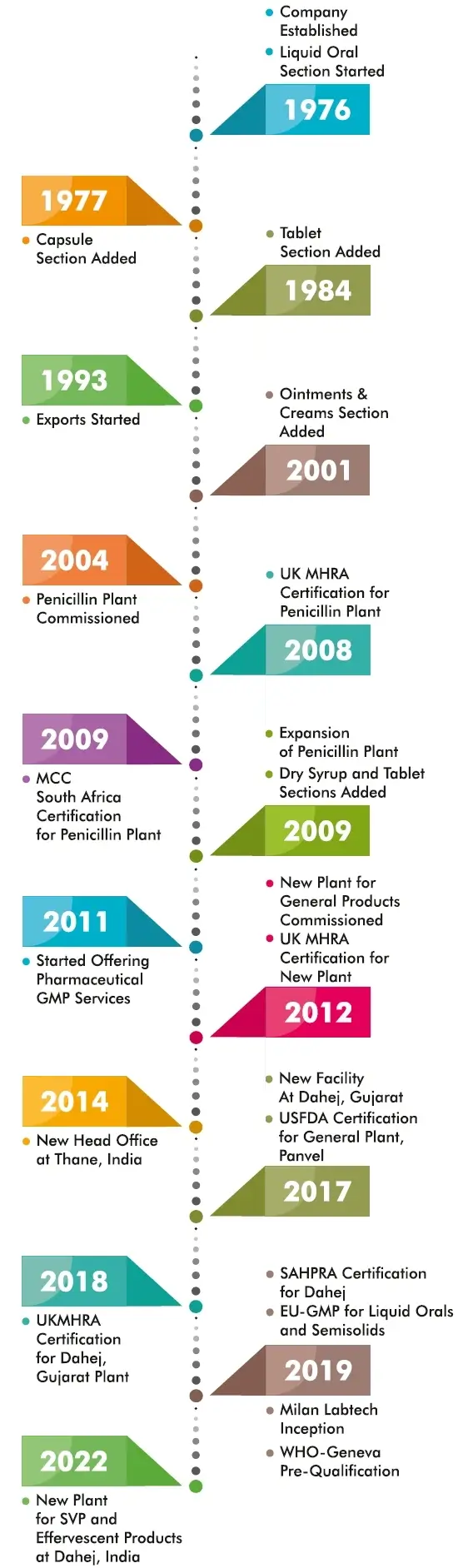
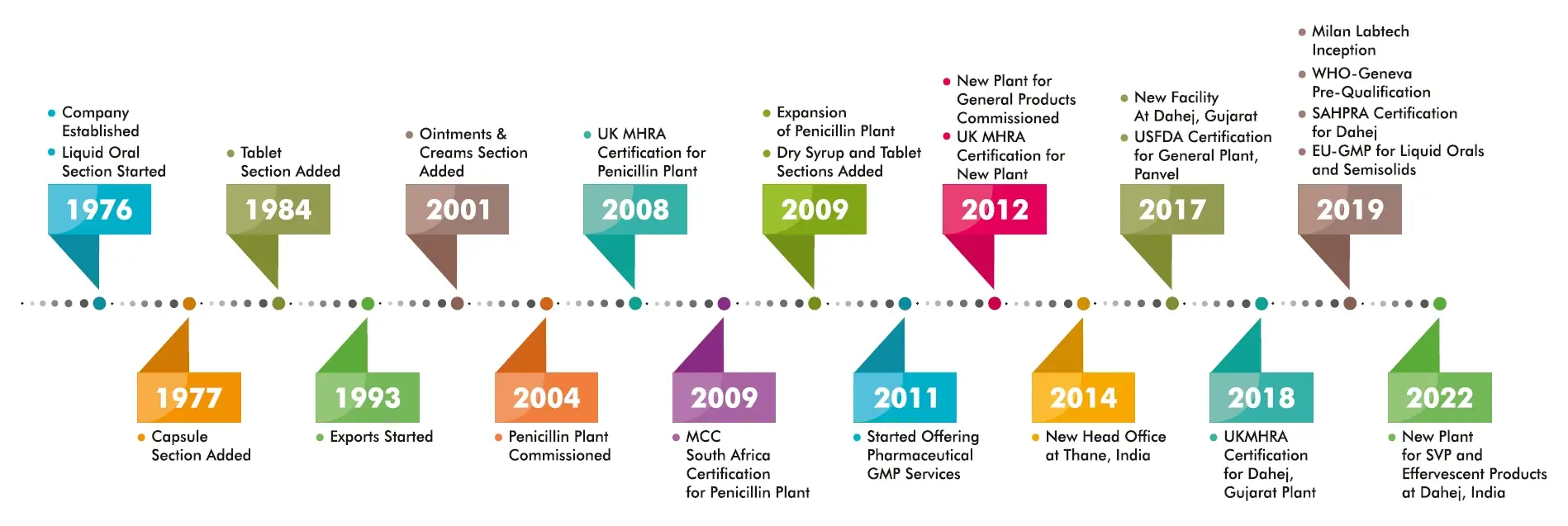
Milan Laboratories (India) Pvt. Ltd. was established in the year 1976 with objective of manufacturing pharmaceutical formulations. A modest beginning, while being morally obligated towards improved human health, liquid orals and tablets were manufactured for the local markets. Supported by initial success, other dosage forms such as capsules, dry syrups and ointments were also added. The gradual expansion of the organization was a series of milestones over the years which included exports, building of a separate facility for penicillin antibiotics and a brand new facility for general products, all replete with modern , state of the art and most current cGMP compliant systems.
The latest cGMP norms were always adopted as a habit and culture. A qualified, experienced, continually updated and committed personnel base and our well maintained facilities has enabled us to achieve quality certifications from WHO, UK-MHRA, South Africa-MCC, most other African and European nations.
Our Organization is ISO 9001-2015 certified.
The Organization has been awarded export awards from Chemexcil(Pharmexil), FICCI and FIEO. The company enjoys star export house status.
Our Customer retention and satisfaction index speaks for our continued success.
Continual improvement is our policy and the company has been adapting to current trends and requirements while adding newer approvals and opening never markets for business.


Quality Policy
The quality control section is well equipped with professionals operating qualified instruments for performing analysis of incoming materials, in process and finished products. This section is responsible for sampling, testing, and release of materials at every stage of manufacturing.
The plants are inspected by the drug regulatory authorities / health authorities of various countries and the constant efforts by the quality operations cell has enabled us to have a very good success rate so far with renewed and valid registrations in several countries.
- We are in the business of development, manufacturing and marketing pharmaceutical formulations and hence we are at all times conscious of the fact that we are ethically committed towards improved human health.
- We shall always strive to achieve customer confidence & satisfaction by manufacturing as per cGMP norms, assuring safety, efficacy and building quality into our products.
- We are committed towards maintaining & reviewing the effectiveness of our Quality Management System through our genuine pursuit of continual improvement.
The quality control section is well equipped with professionals operating qualified instruments for performing analysis of incoming materials, in process and finished products. This section is responsible for sampling, testing, and release of materials at every stage of manufacturing.
The plants are inspected by the drug regulatory authorities / health authorities of various countries and the constant efforts by the quality operations cell has enabled us to have a very good success rate so far with renewed and valid registrations in several countries.
- We are in the business of development, manufacturing and marketing pharmaceutical formulations and hence we are at all times conscious of the fact that we are ethically committed towards improved human health.
- We shall always strive to achieve customer confidence & satisfaction by manufacturing as per cGMP norms, assuring safety, efficacy and building quality into our products.
- We are committed towards maintaining & reviewing the effectiveness of our Quality Management System through our genuine pursuit of continual improvement.
Quality Operations
The quality operations team at Milan Laboratories ensures to provide drugs of consistent quality, purity and efficiency to our patients. In order to achieve this, in house cGMP inspectors ensure that all activities in the manufacturing units are carried out in accordance with cGMP norms.
In process controls, usage of qualified machinery, validated manufacturing processes, validated cleaning procedures, strict environmental & microbiological controls in production and packaging areas, training of personnel, maintaining hygiene and cleanliness, proper record keeping, and so on are some of the controls exercised by this section so as to ensure that quality is built into the final product.
The quality operations team at Milan Laboratories ensures to provide drugs of consistent quality, purity and efficiency to our patients. In order to achieve this, in house cGMP inspectors ensure that all activities in the manufacturing units are carried out in accordance with cGMP norms.
In process controls, usage of qualified machinery, validated manufacturing processes, validated cleaning procedures, strict environmental & microbiological controls in production and packaging areas, training of personnel, maintaining hygiene and cleanliness, proper record keeping, and so on are some of the controls exercised by this section so as to ensure that quality is built into the final product.